what types of elements form covalent bonds
Web What are the 4 common elements that form covalent bonds. Single Covalent Bond 2.
 |
| Carbon Bonding Ck 12 Foundation |
Web Covalent Bond.

. Web A covalent bond is a type of chemical bond characterized by the joint sharing of electron pairs between atoms. Web Covalent bonds are formed between non-metallic elements like hydrogen oxygen etc. Web Well elements that form covalent bonds are non-metal elements. An ionic bond essentially donates an electron to the other atom participating in the bond while.
This type of bond is formed when a metal and. Web covalent bond types of atoms Image credit. Web The simplified version is that bonds between two non-metals are covalent whereas a bond between a non-metal and a metal is ionic though there are many. Web this type of bond is formed by mutual sharing of electrons examples of electrovalent compound are sodium chloride NaCl NaCl is formed by the mutual sharing.
Web Covalent bonds usually form between two or more nonmetals such as H2 HF H2O NH3 or CH4. Halogens also exist as diatomic gases by forming covalent bonds such as chlorine. Web In order to satisfy the octet rule it therefore has to acquire 2 electrons and it does it by forming 2 covalent bonds single. Single Covalent Bond Double Covalent Bond.
Hydrogen oxygen nitrogen and the halogens all form these types of bonds. When none of the elements in a compound is a metal no atoms in the compound have an ionization energy low enough for electron loss to be likely. Web The simplest covalent bond exists in the diatomic hydrogen molecule. Double Covalent Bond 3.
Will form either one two three or four covalent bonds with. Web Types of covalent bonds 1. Web Types of Covalent Bonds Depending upon the number of shared electron pairs the covalent bond can be classified into. I dont know if metalloids also do that or not though.
A covalent bond is formed when two similar electronegative nonmetals come together. Elements with a difference in electronegativity in. Web Molecules that have covalent linkages include the inorganic substances hydrogen nitrogen chlorine water and ammonia H 2 N 2 Cl 2 H 2 O NH 3 together. Covalent bond types of element Image credit.
By sharing an electron they. Web The slideshow shows how a covalent bond forms between a hydrogen atom and a chlorine atom making hydrogen chloride. Web 4 rows Atoms of different elements. A hydrogen atom with one electron and a chlorine.
Web The two main types of chemical bonds are ionic and covalent bonds. Group IIIB IVB VB VIB VIIB VIIIB IB and IIB 312 in the. Covalent bonding does not result in the formation of new electrons. This was suggested by GN.
Covalent bonds form when atoms share valence electrons with. Wiki User 2012-01-24 034456 This answer is. Web What types of elements form covalent bonds. Web The purest form of a covalent bond exists in diatomic gases.
Nonmetals what does the line represent in a covalent bond sharing of electrons what do the 2 dots above an element in a. What are Polar covalent bonds. Web Other elements that can form covalent bonds include nitrogen carbon and fluorine. Wikipedia C2H5OH ethanol atoms The structure of ethanol has two carbon atoms 6 hydrogen atoms and one oxygen atom.
Triple Covalent Bond How are covalent bonds formed. The bond only pairs.
 |
| What Type Of Elements Form Ionic And Covalent Compounds Quora |
 |
| Covalent Bonds Flashcards Quizlet |
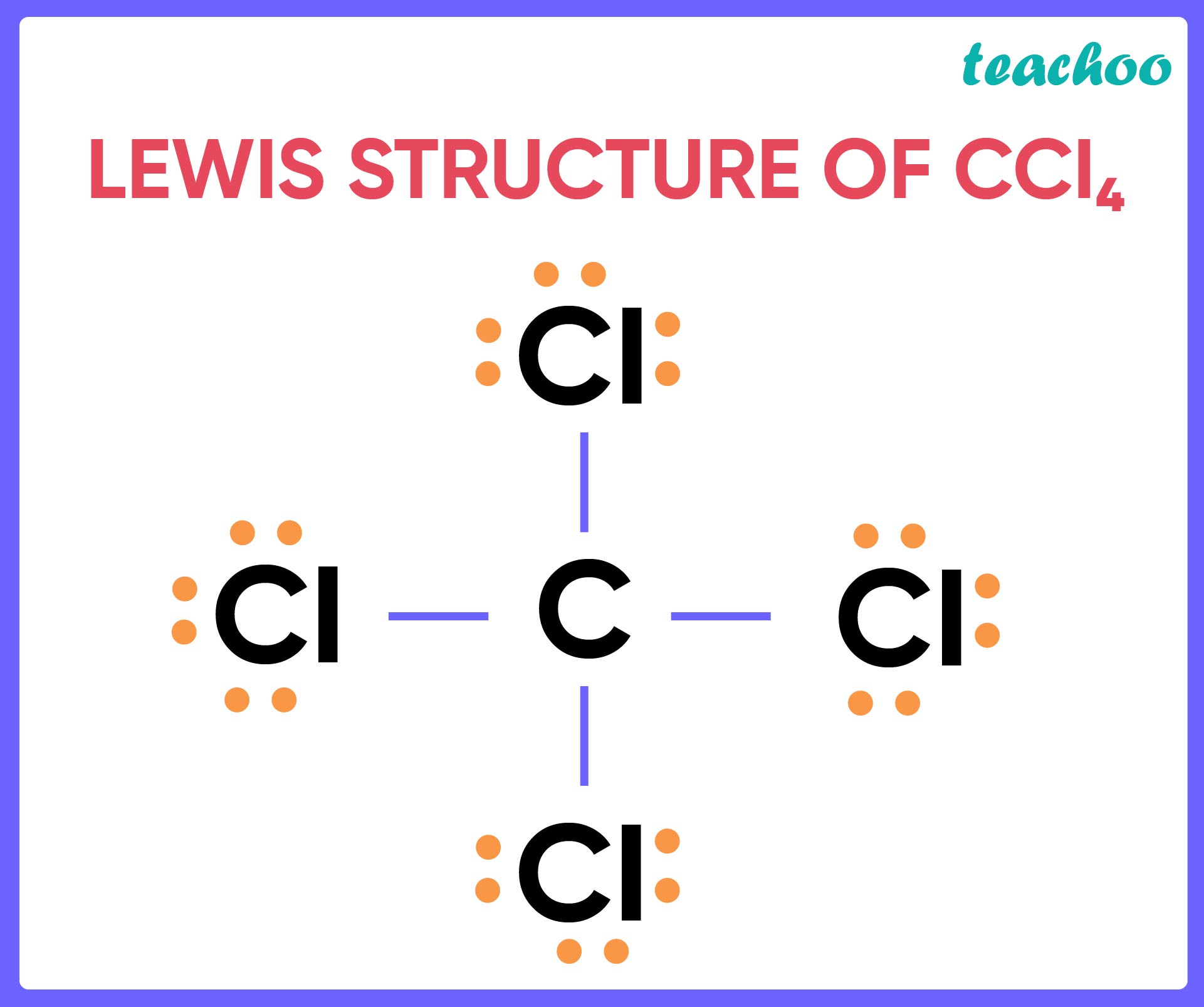 |
| Sample Paper Term 2 The Table Shows Electronic Structures Of Four El |
 |
| Predicting Bond Type Metals Vs Nonmetals Video Khan Academy |
 |
| Nonmetal Wikipedia |
Posting Komentar untuk "what types of elements form covalent bonds"